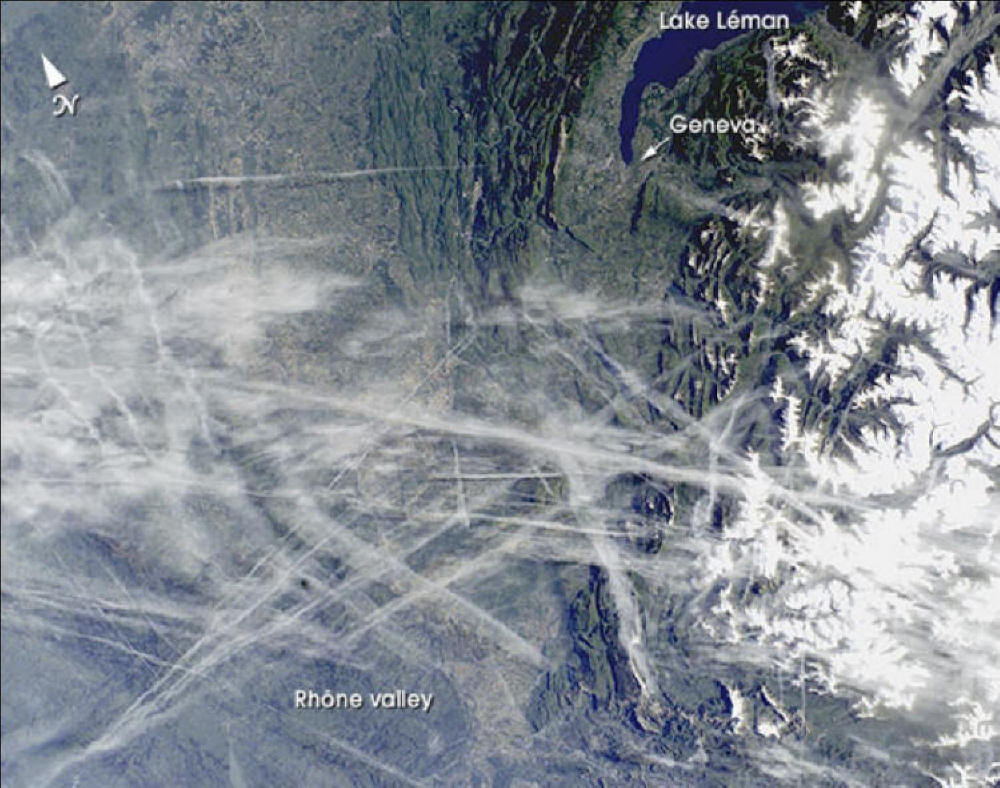
CHEMTRAILS
CHEMTRAILS: Is U.S. Gov't. Secretly Testing Americans 'Again'? Could a strange substance found by an Ark-La-Tex man be part of secret government testing program? That's the question at the heart of a phenomenon called "Chemtrails." In a KSLA News 12 investigation, Reporter Jeff Ferrell shows us the results of testing we had done about what's in our skies. "It seemed like some mornings it was just criss-crossing the whole sky. It was just like a giant checkerboard," described Bill Nichols. He snapped several photos of the strange clouds from his home in Stamps, in southwest Arkansas. Nichols said these unusual clouds begin as normal contrails from a jet engine. But unlike normal contrails, these do 'not' fade away. "This is water and stuff that I collected in bowls. I had it sitting out in my backyard in my dad's pick-up truck," said Nichols as he handed us a mason jar in the KSLA News 12 parking lot back in September after driving down from Arkansas. KSLA News 12 had the sample tested at a lab. The results: A high level of barium, 6.8 parts per million, (ppm). That's more than three times the toxic level set by the Environmental Protection Agency, or EPA. Armed with these lab results about the high levels of barium found in our sample, we decided to contact the Louisiana Department of Environmental Quality. They told us that, 'yes,' these levels are very unusual. But at the same time they added the caveat that proving the source is a whole 'nother matter. We discovered during our investigation that Barium is a hallmark of other chemtrail testing. This phenomenon even attracted the attention of a Los Angeles network affiliate, which aired a report entitled, "Toxic Sky?" There's already no shortage of unclassified weather modification programs by the government. But those who fear chemtrails could be secret biological and chemical testing on the public point to the 1977 U.S. Senate hearings which confirmed 239 populated areas had been contaminated with biological agents between 1949 and 1969. Later, the 1994 Rockefeller Report concluded hundreds of thousands of military personnel were also subjected to secret biological experiments over the last 60-years. But could secret testing be underway yet again? "I'd rather it be something inert and you know something that's not causing any damage but I'd like to know what it is," concluded Nichols. It turns out, until just nine years ago the government had the right, under U.S. law, to conduct secret testing on the American public, under specific conditions. Only a public outcry repealed part of that law, with some "exceptions." Mark Ryan, Director of the Poison Control Center, explained that short term exposure to barium can lead to anything from stomach to chest pains, with long-term exposure causing blood pressure problems. Ryan addressed concerns by chemtrail researchers that barium could be meant to wear down a person's immune system. "Anything that causes ill effects on the body long-term, chronically, is going to affect your ability, it's just constantly working on the body. So from that aspect yeah it's a potential." Ryan told us he's conducted research of his own about secret government testing on the public. But he's still a bit skeptical about chemtrails at the moment, especially considering that his Poison Control Center has seen no calls about barium exposure. http://www.ksla.com/Global/story.asp?s=7339345
WHY BARIUM? Barium–borate–flyash glasses: As radiation shielding materials The Barium Suspension The Element BariumBa Atomic Number: 56 Atomic Weight: 137.327 Melting Point: 1000 K (727°C or 1341°F) Boiling Point: 2170 K (1897°C or 3447°F) Density: 3.62 grams per cubic centimeter Phase at Room Temperature: Solid Element Classification: Metal Period Number: 6 Group Number: 2 Group Name: Alkaline Earth Metal History and Uses: Barium was first isolated by Sir Humphry Davy, an English chemist, in 1808 through the electrolysis of molten baryta (BaO). Barium is never found free in nature since it reacts with oxygen in the air, forming barium oxide (BaO), and with water, forming barium hydroxide (Ba(OH)2) and hydrogen gas (H2). Barium is most commonly found as the mineral barite (BaSO4) and witherite (BaCO3) and is primarily produced through the electrolysis of barium chloride (BaCl2). Barium is used as a getter, a material that combines with and removes trace gases from vacuum tubes. Barium sulfate (BaSO4), a common barium compound, is used as a filler for rubber, plastics and resins. It can be combined with zinc oxide (ZnO) to make a white pigment known as lithophone or with sodium sulfate (Na2SO4) to make another white pigment known as blanc fixe. Stones made from impure barium sulfate glow when exposed to light and will glow in the dark for up to six years if intensely heated in the presence of charcoal. These stones, known as Bologna stones, were discovered near Bologna, Italy in the early 1500s and were thought to possess magical properties by alchemists. Although all barium compounds are poisonous, barium sulfate can be safely ingested since it does not dissolve in water. It is also a good absorber of X-rays and, when swallowed, can be used to produce X-ray images of the intestinal tract. Barium carbonate (BaCO3), another common barium compound, is used in the manufacture of ceramics and some types of glass. It is a component in clay slurries used in drilling oil wells. Barium carbonate is used to purify some chemical solutions and is the primary base material for the manufacture of other barium compounds. Barium forms several other useful compounds. Barium nitrate (Ba(NO3)2) burns with a bright green color and is used in signal flares and fireworks. Barium chloride (BaCl) is used as a water softener. Barium oxide (BaO) easily absorbs moisture and is used as a desiccant. Barium peroxide (BaO2) forms hydrogen peroxide (H2O2) when it is mixed with water and is used as a bleaching agent that activates when wet. Barium titanate (BaTiO3) is used as a dielectric material in capacitors. Barium ferrite (BaO·6Fe2O3) is used to make magnets. |